Azo dyes are important class of synthetic dyes in chemistry. The name azo comes from French word azote, which means nitrogen. IUPAC definition of azo compound is “Derivatives of diazene” (-NH=NH-). Before the discovery of synthetic dyes, people used plant-based dyes such as indigo, saffron, wood flowers for cloth dyeing with no to very little processing. Dyeing was known as early as in the Indus valley period (2500 BC), as is substantiated by the coloured garments of cloths and traces of madder dye found at the Indus Valley Civilization ruins (or remains) at Mohenjodaro and Harappa (3500 BC). Particularly in India and Phoenicia (modern Lebanon), the dyeing has been widely carried out for over 5,000 years.
The first synthetic dye, picric acid, was prepared in 1771 from indigo by Peter Woulfe. It was used in dyeing silk fabric; however due to poor fastness properties, this dye did not have much commercial value.
Next synthetic dye, Mauveine prepared by Willium Henry Perkin in Germany in 1856 catalyzed growth of the synthetic dye industry.

Red and black slip-painted decoration

In 1858, German chemist Peter Griess discovered diazo compound by passing nitrous fumes (corresponds to formula N2O3) into cold alcoholic solution of primary amine. This reaction came to be known as ‘Diazotization’ reaction led to discovery of many more Azo dyes.
Most azo dyes are prepared by azo coupling reaction which is an electrophilic substitution reaction of aryl diazonium cation with another aromatic compound having electron donating group(s).
The preparation is carried out in two steps, the first involving formation of the diazonium salt of a primary amine, using sodium nitrite and hydrochloric acid. This step, known as diazotization reaction, has to be carried out at low temperature. The intermediate (diazonium salt) thus formed is then subjected to the coupling reaction which gives the azo dye.
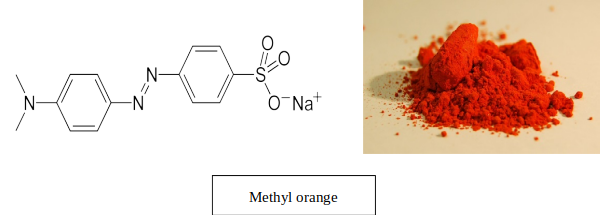
You are already aware about some azo dyes, e.g. Methyl orange. It is synthesized from sulfanilic acid using N,N-dimethyaniline as the coupling agent. Sulfanilic acid is off-white crystalline solid whereas N,N-dimethyaniline is a slightly yellow colored liquid. The product Methyl orange has a bright orange color.
Methyl red is one more example of azo dyes which you probably have used as an indicator in an acid-base titration. It is prepared by diazotization of anthranilic acid followed by coupling reaction with dimethylaniline.
You will enjoy doing the experiment as you will observe the distinct and intense color of the product (azo dye), due to the extended conjugation of π-electrons in its molecules. This synthesis does not require complicated glassware or assembly as you can see in the list of the glasswares. To maintain a low temperature, you will use an ice bath which is available easily in any chemistry lab. Enjoy preparing the azo dye!!!
Prerequisite (Theory)
Students should be familiar with different principles involved in diazotisation reaction
Coupling reaction.
Factors affecting the reaction or reaction conditions
Stoichiometric calculations involved in organic reactions
Techniques
Slow addition of one solution to another with stirring
Filtration of product using suction
Thin Layer Chromatorgraphy (A-guide-to-thin-layer-chromatography-technique)
Reference
- Dye. In Wikipedia. Retrieved March 30, 2021, from https://en.wikipedia.org/wiki/Dye
- Kanetkar, V.R., (2010). Colour: History and Advancements. Resonance – Journal of Science Education, 15(9), 794-803. Retrieved March 30, 2021, from https://www.ias.ac.in/article/fulltext/reso/015/09/0794-0803
- Siva, R. (2007). Status of natural dyes and dye-yielding plants in India. Current Science, 92(7), 916-925. Retrieved March 30, 2021, from http://www.jstor.org/stable/24097672
- Dreher, A. (2009). Fragment of the large vessels found in Harappa (2500 BC). Red and black slip-painted decoration [Photograph]. Wikimedia Commons. https://commons.wikimedia.org/wiki/File:Red_pottery,_IVC.jpg#/media/File:Red_pottery,_IVC.jpg
- Jensen, D. (2005). Yarn drying after being dyed [Photograph]. Wikimedia Commons. https://commons.wikimedia.org/wiki/File:Conner-prairie-yarn drying.jpg#/media/File:Conner-prairie-yarn-drying.jpg
- Other images are taken from the public domain.
Glassware
| ▪ Beaker(100ml) | 3 |
| ▪ Ice bath | 1 |
| ▪ Spatula | 1 |
| ▪ Funnel | 1 |
| ▪ Glass rod | 2 |
| ▪ Measuring cylinder (10mL) | 3 |
| ▪ Filter papers | 2 |
| ▪ Wash bottle | 1 |
| ▪ Pre weighed butter paper | 1 |
| ▪ TLC chamber | 1 |
| ▪ Capillary tubes | 3 |
| ▪ TLC plates | 1 |
| ▪ Fusion tubes | 3 |
Chemicals
| ▪ NaNO2 solution | 10 mL |
| ▪ Conc. HCl | 3 mL |
| ▪ 10 % NaOH | 7 mL |
| ▪ Aniline | 1 mL |
| ▪ β-napthol | 0.75 g |
| ▪ Starting compound for TLC | |
| ▪ Acetone for dissolution, | |
| ▪ Eluent for TLC (hexane :Ethyl acetate) |
Hazard Symbols
Corrosive to skin ![]()
Oxidizing ![]()
Toxic ![]()
Irritant ![]()
Harmful to environment ![]()
Flammable ![]()
The history of dyeing is divided into two great periods, the “pre-aniline,” extending to 1856 and the “post-aniline” period. The former was characterized by a rather limited range of colors that were based on dye-producing animals and plants. The main vegetable dyes available were extracted from roots of madder plant (a perennial climbing plant with evergreen leaves and small pale yellow flowers found in Asia and Europe), producing a brilliant red and leaves of the indigo plant (India), yielding the blue dye still used today in jeans.
Aniline became available from coal tar in the 19th century and in 1856, William Henry Perkin at the age of 17 used it in the synthesis of “Mauveine”. Since then, synthetic dyes started replacing natural dyes.
This laboratory task involves preparation of an organic dye, namely, Sudan-I from aniline. The first step of reaction involves diazotization of aniline using sodium nitrite solution (eq 1), whereas the second step involves coupling of the diazonium salt with β-naphthol (eq 2).
Ar-NH2 + NaNO2 + 2HCl [icon icon=icon-arrow-right size=14px color=#000 ] Ar-N2+Cl– + NaCl + 2H2O …………1
Ar-N2+ Cl– + Ar’H [icon icon=icon-arrow-right size=14px color=#000 ] Ar-N=N-Ar’ + HCl ….……… 2
Procedure
a) Preparation of diazonium salt
- A vial containing 1 mL of aniline is supplied to you. Transfer the entire content of the vial to a clean 100 mL beaker. Using a measuring cylinder, add 2.5 mL of conc. HCl and 5 mL of distilled water to this beaker. Stir the solution with a glass rod to obtain a clear solution. Cool this solution in an ice-bath for 5 to 10 minutes.
- Chilled sodium nitrite solution is supplied to you on your table. Add 5 mL of the sodium nitrite in a dropwise manner to the above aniline solution with constant stirring. The addition should be done in cold condition only.
b) Coupling Reaction
- In another clean beaker transfer the entire content of the vial containing β-naphthol. Add 5 mL of NaOH solution and 5 mL of distilled water. Stir well with a glass rod to obtain a clear solution. Cool this solution in an ice-bath to 0°C.
- Add dropwise the ice cold diazotised solution (prepared in Part a) into the ice cold solution of β-naphthol with constant stirring.
- Filter the precipitate using a Buchner funnel and under suction. Inform the laboratory expert when the filteration is over. After filtration, the precipitate should be handed over to laboratory expert for drying.
- The precipitate will be handed back to you after it is dried. Carefully transfer the product on a pre-weighed butter paper supplied to you. Take the product for weighing to the laboratory expert.
- Record the TLC of the final product after it is weighed.
Dissolve a small crystal of Sudan I, Aniline and β-naphthol in a small quantity of acetone in a sodium fusion tube respectively. Develop the TLC Plate using the solvent and visualize the spots under UV light. Calculate the Rf value using the formula given below and record the result in the answer sheet.
- The mass of the product.
- Calculate theoretical yield (based on aniline) in g :
- The yield obtained as a percentage of the theoretical yield:
- Colour of the product obtained: Dark Brown Yellow Orange red Red Any other
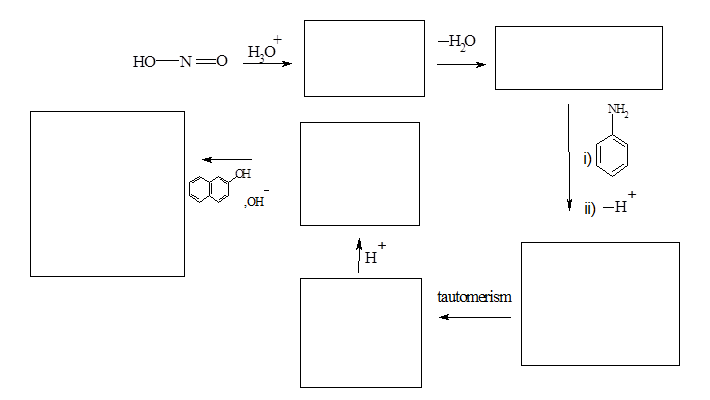
- Mechanism of reactions involved in synthesis of Sudan-I is given below. Draw the structures of intermediates and sudan-I.
- Azo compounds can be reduced to amines by a variety of reagents including SnCl2/HCl is one of them.
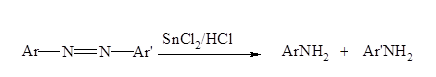
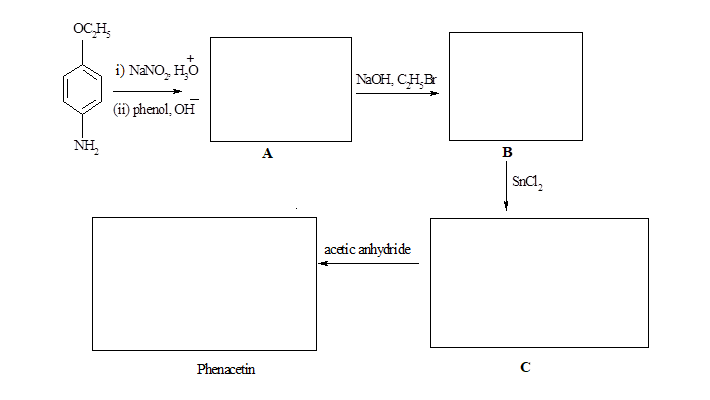
This reduction can be useful in the synthesis of phenacetin (an analgesic). Give the structure of phenacetin and the intermediates A,B and C.
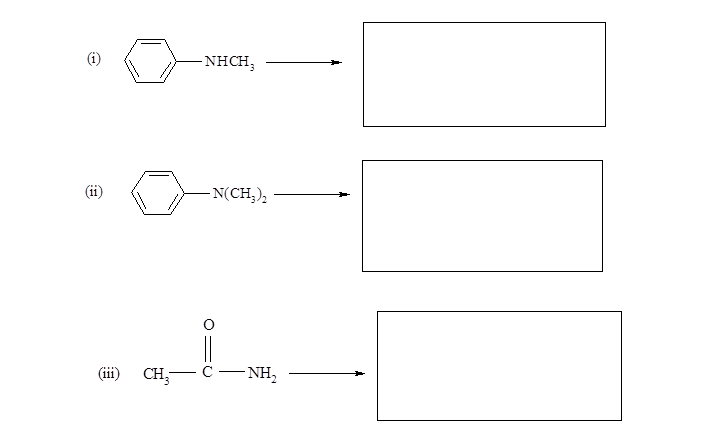
- Draw the structures of the products obtained when the following compounds are treated with NaNO2/HCl at 0oC.
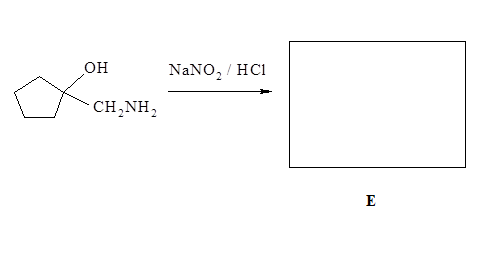
8.Treatment of the following amino compound with NaNO2/HCl at 0 to 5oC gives compound E which gives a positive 2,4-DNP test. Draw structure of E
This video is shot in the chemistry laboratory of HBCSE and can be used for educational purposes