The current experiment is about determination of Al(III) and Mg(II) in a given sample of antacid tablet using complexometric titrations. The experiment uses pH and masking agents, to determine these metal ions using disodium salt of ethylenediammine tetraacetic acid (Na2EDTA) as a titrant.
Prerequisite (Theory)
- EDTA and its equilibria, effect of pH on EDTA equlibria
- Metallochromic indicators in EDTA titration
- pH and masking agents in complexometric titration
- back titration
Techniques/Skills involved
- Use of pipette bulb: Guide to use a Pipette aid
- Titration technique
- Preparation of solution in standard flask
References
Jeffery, G.H., Bassett, J., Mendham, J., Denney, R.C., (1989). Vogel’s Textbook of Quantitative Chemical Analysis. John Wiley & Sons Inc, New York, 5th edition (309-322).
Skoog, D.A., West, D., Holler, F.J., (1995). Fundamental of Analytical Chemistry. Harcourt Asia PTE Ltd, Singapore, 7 edition, (400-422)
These textbooks presents the necessary discussions about general theory of complexometric titration including the role of various parameters such as pH, masking agents, metallochromic indicators etc.
https://chem.libretexts.org/Textbook_Maps/Analytical_Chemistry_Textbook_Maps/Map%3A_Analytical_Chemistry_2.0_(Harvey)/09_Titrimetric_Methods/9.3%3A_Complexation_Titrations (accessed on September 2017)
Glassware
| ▪ Burette (25mL) | 2 |
| ▪ Conical flasks (250mL) (100 mL) | 4 1 |
| ▪ Dropper | 2 |
| ▪ Funnel | 1 |
| ▪ Filter paper | 1 |
| ▪ Hot plate | 1 |
| ▪ Measuring cylinder (10mL) | 1 |
| ▪ Measuring cylinder (25mL) | 1 |
| ▪ Pipette 10 mL | 1 |
| ▪ Pipette bulb | 1 |
| ▪ pH papers | 3 |
| ▪ Standard flask (100 mL) | 1 |
| ▪ Wash bottle | 1 |
Chemicals
| ▪ Sample, 0.500 g | |
| ▪ 0.0500 M Na2EDTA | |
| ▪ Buffer pH 10, 120 mL | |
| ▪ Triethanolamine (TEA), 10.0 mL |
| ▪ 0.0500 M ZnSO4 solution, 20.0 mL | |
| ▪ Calmagite indicator |
(molarity of Na2EDTA and ZnSO4 can be provided later)
Hazard Symbols
Corrosive to skin ![]()
Oxidizing ![]()
Toxic ![]()
Irritant ![]()
Harmful to environment ![]()
Flammable ![]()
Antacids are commonly used to get relief from acid indigestion. Usually, these neutarlize excess acids formed in the body. These are generally categorized in two types: i) Chemical antacid that uses the concept of neutralizing the gastric acid. For example, sodium bicarbonate ii) absorptive antacids which adsorb the acid, these generally include aluminium and magnesium salts and calcium carbonate.
Ref: Shui-Ping Yang and Ruei-Ying Tsai ; Complexometric Titration of Aluminum and Magnesium Ions in Commercial Antacids. An Experiment for General and Analytical Chemistry Laboratories, Journal of Chemical Education 2006 83 (6), 906.DOI: 10.1021/ed083p906
https://www.encyclopedia.com/medicine/drugs/pharmacology/antacid
In this experiment, we will estimate the amount of Al (III ) and Mg (II) ions present in a antacid tablet. This tablet contains both Al(III) and Mg(II) as hydroxides.
Initially, you will dissolve the antacid sample with an acid and prepare a solution of known dilution. Then a fixed volume of this solution will be taken for titration purpose. You will be determining Al(III) and Mg(II) using complexometric titration. In Titration I you will determine the total metail ion content, that is, Al(III) and Mg(II), whereas in Titration II you will determine Mg(II) content using another a masking agent.
Complexometric titrations also depend on the formation constants of the complexes that are getting formed in the titration. Given below is the graph of log of formation constants of the complexes of the metal ions with Na2EDTA, as a function of pH. The two metal ions that you will be estimating are indicated on the graph.
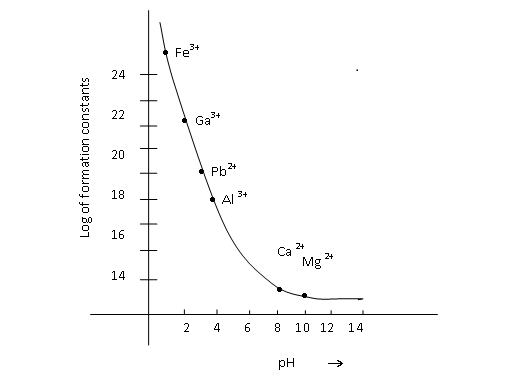
Adapted from (Skoog, D.A., West, D., Holler, F.J., (1995). Fundamental of Analytical Chemistry. Harcourt Asia PTE Ltd, Singapore, 7 edition)
Procedure
Preparation of sample solution for analysis
A vial containing the sample in powder form is supplied you. Carefully empty the entire content of the vial in a 250 mL conical flask. Add 50.0 mL of water and 3.0 mL of 6.0 M HCl solution. Transfer the flask to a hot plate and gently boil the solution for half an hour. Remove the flask from the hot plate and allow it to cool for 5 minutes.
Filtration of the sample solution
You will be removing undissolved content (especially binders) present in the tablet by filtration. You will be filtering the solution in 100 mL standard flask and using distilled water. The filtrate is diluted to 100 mL.
Determination of the total Al and Mg content (Titration I)
- Pipette 10.0 mL of the diluted solution in a 250 mL conical flask and add 30.0 mL of water. Then add 10.0 mL of buffer solution (pH 10) followed by 40.0 mL of supplied Na2EDTA solution.
- Boil the mixture for 5 minutes on a hot plate. Remove the flask from the hot plate (use gloves to hold the hot flask), check the pH of the solution, if it is not pH 10 then add 5.0 mL of buffer solution to it. Then add to it 5 drops of Calmagite The solution should turn blue. If not, then add another 5 mL of Na2EDTA solution and boil until colour changes to blue. Then titrate the hot solution against standardized Zn(II) solution till the colour changes to purple.
- Take at least two more readings in similar manner. Enter your reading in the answer sheet.
Determination of the Mg(II) content (Titration II)
- Pipette 10.0 mL of the given solution in a 250 mL conical flask and dilute it with 30 mL of water.
- Add 25.0 mL of pH 10 buffer followed by addition of 3.0 mL of Triethanolamine. Keep shaking the solution thoroughly for 1 minute and allow it to stand for a while. You will get almost a clear solution at this point.
- Add 5 drops of Calmagite indicator and swirl the content. The solution should be wine red in colour.
- Titrate the solution with the given standard Na2EDTA solution until the colour changes to pure blue at the end point.
- Take at least two more readings in a similar manner. Enter your results in the answer sheet.
Concentration of Na2EDTA solution: ………..M
Concentration of Zn (II) solution:…………M
| Titration I | Titration II | |||||
| Trial 1 |
Trial 2 |
Trial 3 |
Trial 1 |
Trial 2 |
Trial 3 |
|
| Initial burette reading (mL) | ||||||
| Final burette reading (mL) | ||||||
| Volume of Na2EDTA(mL) | ||||||
- Write the balanced chemical equations for the reactions of Al(III) and Mg(II) with Na2EDTA. (Use the symbol Na2H2Y for Na2EDTA.)
- Calculate the Aluminum hydroxide and Magnesium hydroxide content in grams in the total diluted sample. Show your calculations for any one set of reading. (Show main steps in your calculation).
1.The colour change at the end point (blue to purple) in the Titration I is due to
[Mark X in the correct box.]
- the formation of the metal-indicator complex.

- the release of the free indicator from the metal-indicator complex.

- the formation of metal-EDTA complex.

2. If you have to determine the Aluminium content alone then at what optimum pH the titration should be performed? Between Magnesium and Aluminum which forms stronger complex with Na2EDTA?
3. The solution needs to be boiled in Titration I whereas Titration II is performed at room temperature.
This indicates [Mark X in the correct box.]:
- the formation of Aluminium-EDTA complex is kinetically slow

- the formation of Magnesium-EDTA complex is kinetically slow

- the formation of indicator-EDTA complex. is kinetically slow

4. The procedure states that Titration II should be performed as rapidly as possible.
This indicates [Mark X in the correct box.]:
- the Aluminium-TEA complex is very stable

- the Magnesium-EDTA complex is not stable

- the indicator-Na2EDTA complex. is stable

This video is shot in the chemistry laboratory of HBCSE and can be used for educational purposes
This video is shot in the chemistry laboratory of HBCSE and can be used for educational purposes
This video is shot in the chemistry laboratory of HBCSE and can be used for educational purposes