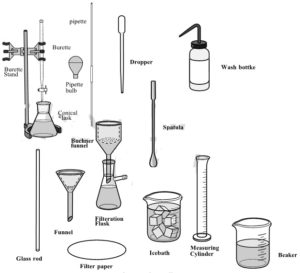
In this experiment, you will prepare a octahedral complex of Cu(II) in aqueous medium. The synthesized complex is further analyzed for its copper and ammonia content. Copper is determined iodometrically and ammonia content is determined by back titration method.
Prerequisite:
- Knowledge about coordination complex and their geometry
- Different types of titration
- Acid-base indicators/ Theory of acid base titration
Techniques and Skills:
- Titration (Using burette, pipette, taking proper volumetric measurements)
- Filtration
- Use of weighing balance
References
Jeffery, G.H., Bassett, J., Mendham, J., Denney, R.C., (1989). Vogel’s Textbook of Quantitative Chemical Analysis. John Wiley & Sons Inc, New York, 5th edition, chapter 10, (pp. 360-416)
This book gives a detailed account of the theoretical concepts of oxidation-reduction reactions using different examples. Writing half cell equations followed by complete balanced equations, titration curves, determination of the end point of titration, choice of indicators and examples of typical redox titrations carried out in the chemistry laboratories are dealt in depth.
Skoog, D.A., West, D., Holler, F.J., (1995). Fundamental of Analytical Chemistry. Harcourt Asia PTE Ltd, Singapore, 7 edition, chapter 17, (pp. 360-385)
This book also deals with the concepts of oxidation-reduction reactions, writing balanced redox equations, discussion of titration curves, determination of end point of titration, choice of indicators and examples of few redox titrations (given at the end of the book separately).
https://chem.libretexts.org/Demos%2C_Techniques%2C_and_Experiments/General_Lab_Techniques/Titration/Acid-Base_Titrations (accessed on April 2018)
https://chem.libretexts.org/Textbook_Maps/Analytical_Chemistry_Textbook_Maps/Map%3A_Analytical_Chemistry_2.0_(Harvey)/09_Titrimetric_Methods/9.4%3A_Redox_Titrations (accessed on April 2018)
https://chem.libretexts.org/Core/Inorganic_Chemistry/Coordination_Chemistry (accessed on April 2018)
Synthesis of complex
| ▪ Beaker (100 mL) | 1 |
| ▪ Dropper | 1 |
| ▪ Filter paper | 2 |
| ▪ Glass rod | 1 |
| ▪ Ice bath | 1 |
| ▪ Measuring cylinders (10 mL) (25 mL) | 1 1 |
| ▪ Spatula | 1 |
Analysis of complex
| ▪ Conical flask (100 mL) (250mL) | 2 4 |
| ▪ Burette (25mL) | 2 |
| ▪ Measuring cylinder (10ml) (25ml) | 2 1 |
| ▪ Dropper | 1 |
| ▪ Funnel | 1 |
| ▪ Wash bottle | 1 |
Chemicals Required
| ▪ 6 M NH3 solution, 25 mL | |
| ▪ Ethanol, 30 mL | |
| ▪ CuSO4.5H2O, 5 g | |
| ▪ 1 M H2SO4, 5 mL | |
| ▪ NaOH (for titration), 50 mL | |
| ▪ 4 M CH3COOH, 40 mL |
| ▪ 2 M NaOH, 15 mL | |
| ▪ Na2S2O3 solution, 100 mL | |
| ▪ KIO3 0.054 g /vial (2 vials) (Mol wt: 214) | |
| ▪ KI, 0.5 g / vial (2 vials) | |
| KI, 1 g / vial (2 vials) (for Cu estimation) |
Hazard Symbols
Corrosive to skin ![]()
Oxidizing ![]()
Toxic ![]()
Irritant ![]()
Harmful to environment ![]()
Flammable ![]()
In this experiment you will prepare a co-ordination complex of Cu (II) and ammonia in Part A. In Part B, you will perform standardization titration of Na2S2O3 solution to calculate its molarity. This titrant is used to estimate copper content of your complex. You will also perform blank reading for HCl solution in this subpart. This blank reading of HCl will be required to calculate ammonia content of the prepared complex. In Part C of the experiment, you will be analyzing the complex synthesized by you for its copper and ammonia contents using titration technique.
PART A
Synthesis of the complex
You are supplied with 5.00 g of CuSO4.5H2O in a sample vial and cold NH4OH solution. Carefully transfer the entire amount of Cu (II) sulphate in a 100 mL beaker. Using a measuring cylinder, add 15 mL of the given cold ammonia solution to the beaker. Keep the beaker in ice bath and do remember that the entire synthesis should be done in cold condition only. Stir the mixture for 2-3 minutes, using a glass rod. To the same solution, add another 5 mL of the cold ammonia solution with stirring. To this solution, slowly add 20 mL of cold ethanol with constant stirring. The addition of entire ethanol content should be done in a dropwise manner. The product will be precipitated in the beaker and you have to take it for filtration. The filtration is done using a Buchner funnel and using suction. You should use cold ethanol solution (maximum 15 mL) for transferring and washing the precipitate on the Buchner funnel. The ethanol needed for washing purpose is kept near the filtration assembly. The laboratory expert will guide you in this regard. After filtration, carefully take the product back to your table. Allow the product to dry for 30 to 40 minutes. At the end of this time interval, carefully transfer the product on the pre-weighed butter paper supplied to you. Take the product for weighing to the laboratory expert.
While the product is drying, perform the following titrations
- Standardization of Na2S2O3 solution
- Blank titration for HCl solution
PART B
Standardization of supplied Na2S2O3 solution
Transfer the content of one vial of KIO3 (0.054g) and one vial of KI (0.500 g) in a 100 mL conical flask. Add 25 mL of distilled water and 1 mL of 1 M H2SO4 solution. At this stage the solution will be brown in colour. Immediately start titrating the solution with Na2S2O3 solution till its colour changes to light yellow. At this point, add 1 mL of starch indicator. The reaction solution will be blue in colour. From this point, addition of Na2S2O3 should be done gradually (drop wise) and with constant shaking. At the end point, the solution will become colorless. Record your readings in the appropriate table provided in the answer sheet. Perform yet another titration in a similar manner. If the two readings are differing widely, then only take the third reading.
Blank titration for HCl
Take 20 mL of supplied HCl solution with the help of a burette. Add 30 mL of distilled water and 3 to 4 drops of methyl red indicator. Titrate the solution with standardized NaOH solution. Perform yet another titration in similar manner. If the two readings are differing widely, then only take the third reading.
PART C: Analysis of the prepared complex
Determination of the Cu content
For this titration, weigh 0.450 g of the prepared product in duplicate. The laboratory expert will help you in this regard.
- Transfer 0.450g of weighed sample in a clean conical flask.
- Add 50 mL of distilled water and add 2 M NaOH solution in drop wise manner until the sample solution becomes slightly turbid.
- Then add 10 mL of 4 M acetic acid and 1 vial of solid KI powder. At this stage the solution will be brown in color.
- Start titrating the solution against the standardized Na2S2O3 solution till its color changes to light yellow. At this point, add 1 mL of starch indicator. The solution will be blue in colour. Continue the titration with shaking till the solution becomes colorless.
- In a similar manner, perform the titration the other sample weighed by you. Enter your results in the answer sheet. If the two readings are differing widely, you should take one more reading. Record your readings
Determination of the ammonia content
For this titration, weigh 0.0850 g of the prepared product in duplicate. The laboratory expert will help you in this regard.
- Transfer 0.0850g of the weighed sample to a clean conical flask.
- Add 50 mL of distilled water and 20 mL of the supplied 0.1 M HCl solution (use burette to take HCl solution).
- Add 4 drops of Alizarin Red S indicator and 4 drops of Bromocresol green indicators.
- Titrate excess of HCl solution against the supplied NaOH solution until the solution changes to blue colour.
- In similar manner, perform the titration with another sample weighed by you. Enter your results in the answer sheet. If the two readings are differing widely, you should take one more reading.
- The blank titration for HCl is already done by you.
Standardization of Na2S2O3 solution
| I | II | III | |
| Initial burette reading (mL) | |||
| Final burette reading (mL) | |||
| Difference (mL) |
Calculate the molarity of supplied Na2S2O3 solution. Show the main steps of your calculations.
Blank Titration (for HCl)
| I | II | III | |
| Initial burette reading (mL) | |||
| Final burette reading (mL) | |||
| Difference (mL) |
Analysis of complex
Calculate
| Copper Titration | Ammonia Titration | |||||
| I | II | III | I | II | III | |
| Initial burette reading (mL) | ||||||
| Final burette reading (mL) | ||||||
| Difference (mL) | ||||||
The amount of Copper and ammonia (amine) in grams for any one reading. (Show the main steps in your calculation).
Mass of the complex
The yield obtained as a percentage of the theoretical yield
Colour of the product.
Questions related to experiment performed
Write balanced chemical equations for reaction between KIO3 and KI in acidic medium.
Write the balanced chemical equation for the reaction involved in the titration of this solution with Na2S2O3.
Write the balanced chemical equation for the reaction involved in the titration of ammine.
During estimation of copper from the complex, initially NaOH is added followed by acetic acid. Explain the role of these reagents.
During the estimation of copper solid KI is added after which the solution turns brown in colour. Write the balanced chemical equation for the reaction that takes place when solid KI is added.
Calculate the amount of copper and ammonia (amine) in grams for any one reading. (Show the main steps in your calculation).
Calculate the sulphate and water content of the complex.
The molar ratio of copper: amine: sulphate: water in the given complex is
Using the molar ratio of copper and amine calculated by you, write the balanced chemical equation/s for reaction/s involved in the formation of the complex.
This video is shot in the chemistry laboratory of HBCSE and can be used for educational purposes
This video is shot in the chemistry laboratory of HBCSE and can be used for educational purposes
This video is shot in the chemistry laboratory of HBCSE and can be used for educational purposes